- Posted
- Categories
-
- OpenSAFELY
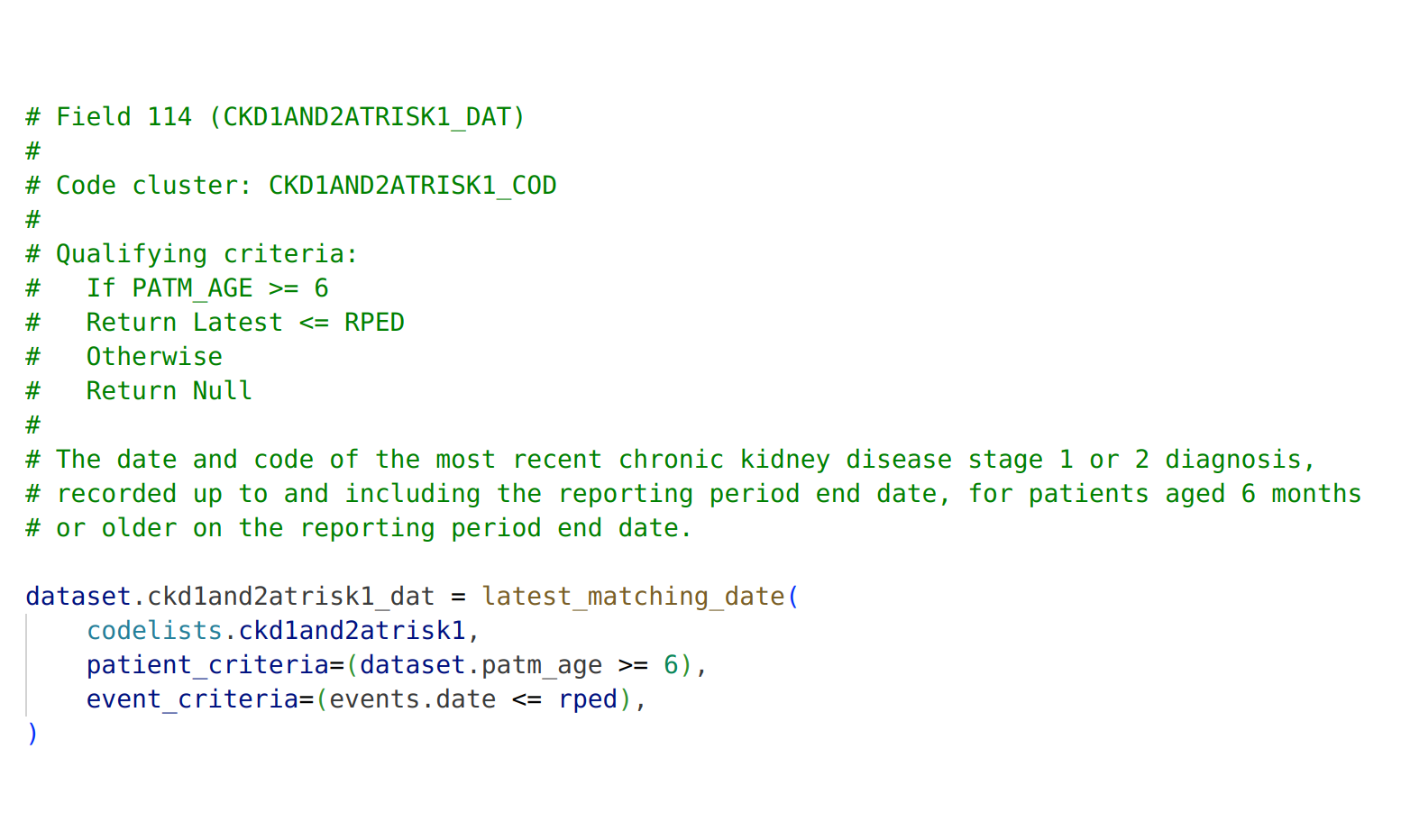
Unlocking Discoveries Safely: Using the primary care data of 59 million patients to determine the impact of the COVID-19 pandemic on antipsychotic prescribing in at-risk populations
In this blog post Millie Green shares some of the findings from a federated analysis carried out using OpenSAFELY.