
- Posted
- Categories
-
- OpenSAFELY
Introduction to the Epidemiology Team
Here we introduce the Bennett Institute’s Epidemiology team, explaining who they are, what they do, and why it is important.
Latest news and views from around the Bennett Institute

Here we introduce the Bennett Institute’s Epidemiology team, explaining who they are, what they do, and why it is important.
Our OpenSAFELY study comparing the Pfizer and Moderna vaccines for protecting against covid-19 during the first booster programme in England is now published open-access in the BMJ.

Here we introduce the Bennett Institute’s Clinical Informatics team, explaining what they do and how their experience in NHS clinical practice helps them do this.

Hello, from the Bennett Institute’s Data Team! In this short blog post, we’ll describe our mission, our backgrounds in industry and academia, and what we’re working on now.

Taking some time to describe the co-piloting programme in numbers, to give an idea of the scale of this programme, who our co-pilots are and who we are working with

Better and more efficient health science with reusable pipelines and cross-disciplinary expertise.

Taking a deeper look at the ‘Safe Outputs’ dimension of this framework, and how it is applied in OpenSAFELY.
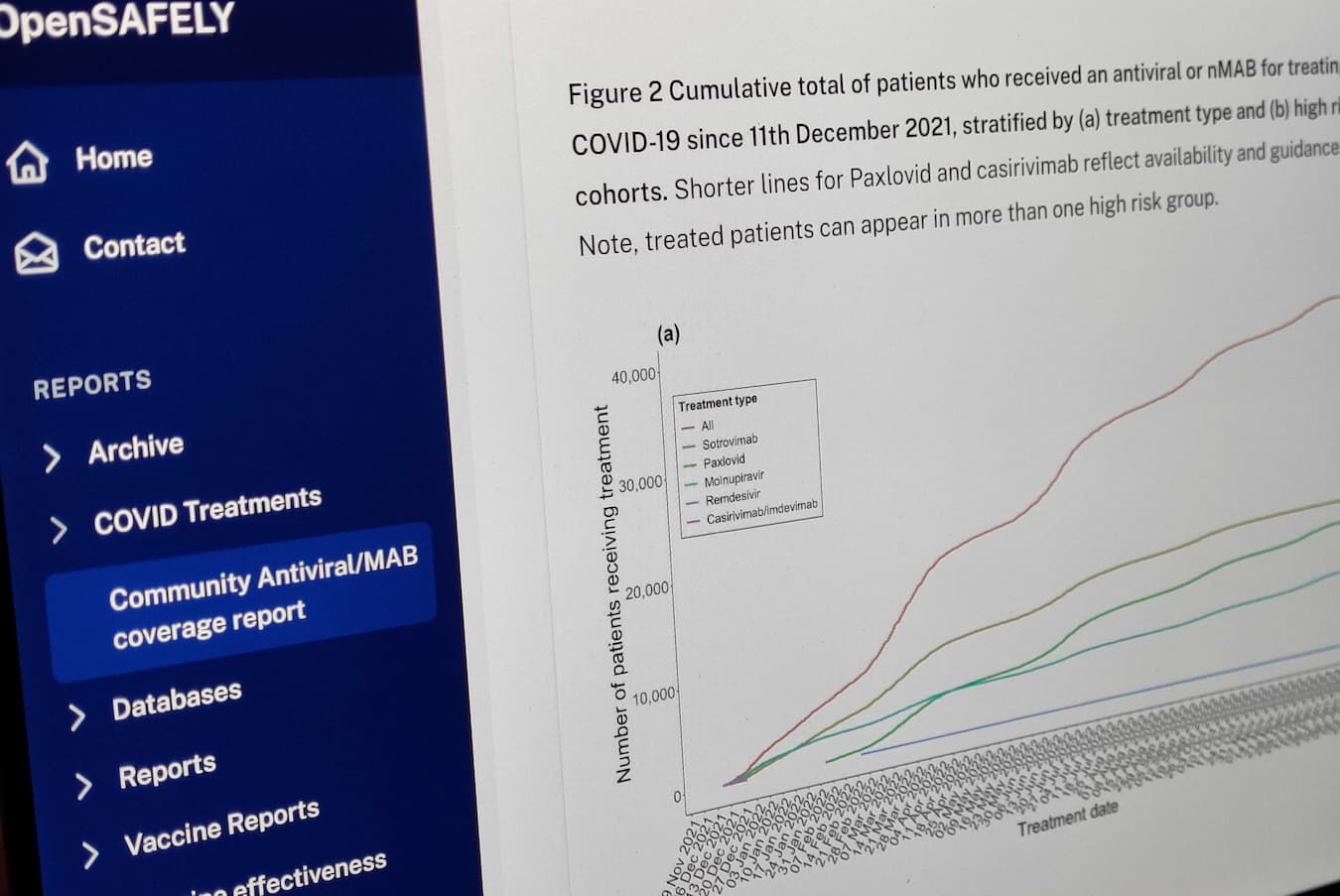
Here we outline some of the research that has been produced with OpenSAFELY, as well as the features that make it such a great platform for research.
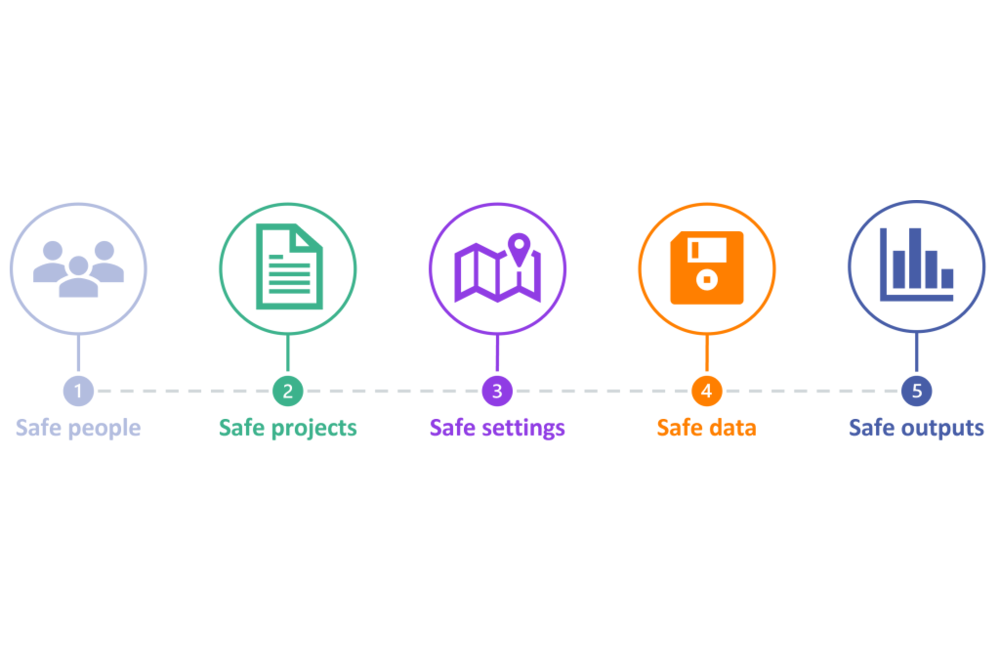
The Fives Safes framework is a popular framework for designing safe and efficient data access systems that has been adopted by a range of Trusted Research Environments (TREs) across the UK.
On 7th May 2020, the OpenSAFELY Collaborative pre-printed the world’s largest study into factors associated with death from COVID-19, based on an analysis running across the full pseudonymised health records of 40% of the English population.

In order to tackle health inequalities NHS England have recently launched the Core20PLUS5 initiative to help local areas identify and reduce inequalities across key areas.

We recently presented on behalf of our team (grp-EHR) at an open science workshop, hosted by the MRC Biostatistics Unit at the University of Cambridge. This was a great opportunity for us to reflect on our team’s experiences using OpenSAFELY, and more broadly embracing open and team science approaches to our work.

As a team of co-pilots, we regularly take time to discuss how co-piloted projects are going and what we can do to help pilots and co-pilots make the most of their time together.

Rachel Seeley is the Head of Analytics at PrescQIPP CIC and has recently completed a project in OpenSAFELY on the safer use of anticoagulant medication. In this guest blog, Rachel describes her experience with using the OpenSAFELY platform and our co-pilot programme.

One of our key aims at Bennett Institute for Applied Data Science is to develop novel data-driven approaches to interpret our health care data. We want to use data science to identify patterns in the data that afford us a new, unbiased perspective on how well the NHS is working and how it could be better.

Bennett Institute resident pharmacist Brian MacKenna sets out some brief information on how we currently build medicines codelists on OpenSAFELY.

The Clinical Trial Information System (CTIS) is the European Union’s new registry set to fully replace the existing EU Clinical Trials Registry next year. Nick DeVito decided to take a spin through the registry and record some of his initial thoughts.

In June 2022, the NHS published its new data strategy Data Saves Lives. The strategy is a broad ranging document, covering seven ‘key areas’.