
- Posted
- Categories
-
- OpenSAFELY
Help us improve research into COVID-19 Vaccination
Could you help us to improve the research that we are doing into COVID-19 vaccination?

Could you help us to improve the research that we are doing into COVID-19 vaccination?

We’ve launched a new project to understand who received which COVID-19 booster shots, and when, and the impacts of this on health outcomes.

It’s not too late to register for our 2nd Annual OpenSAFELY Symposium, which is happening in London on 25/26 November.

We briefly summarise all the recent big and exciting developments in hospital medicines data.

Someone on the radio was musing about using retail data for health - we have some thoughts on that.
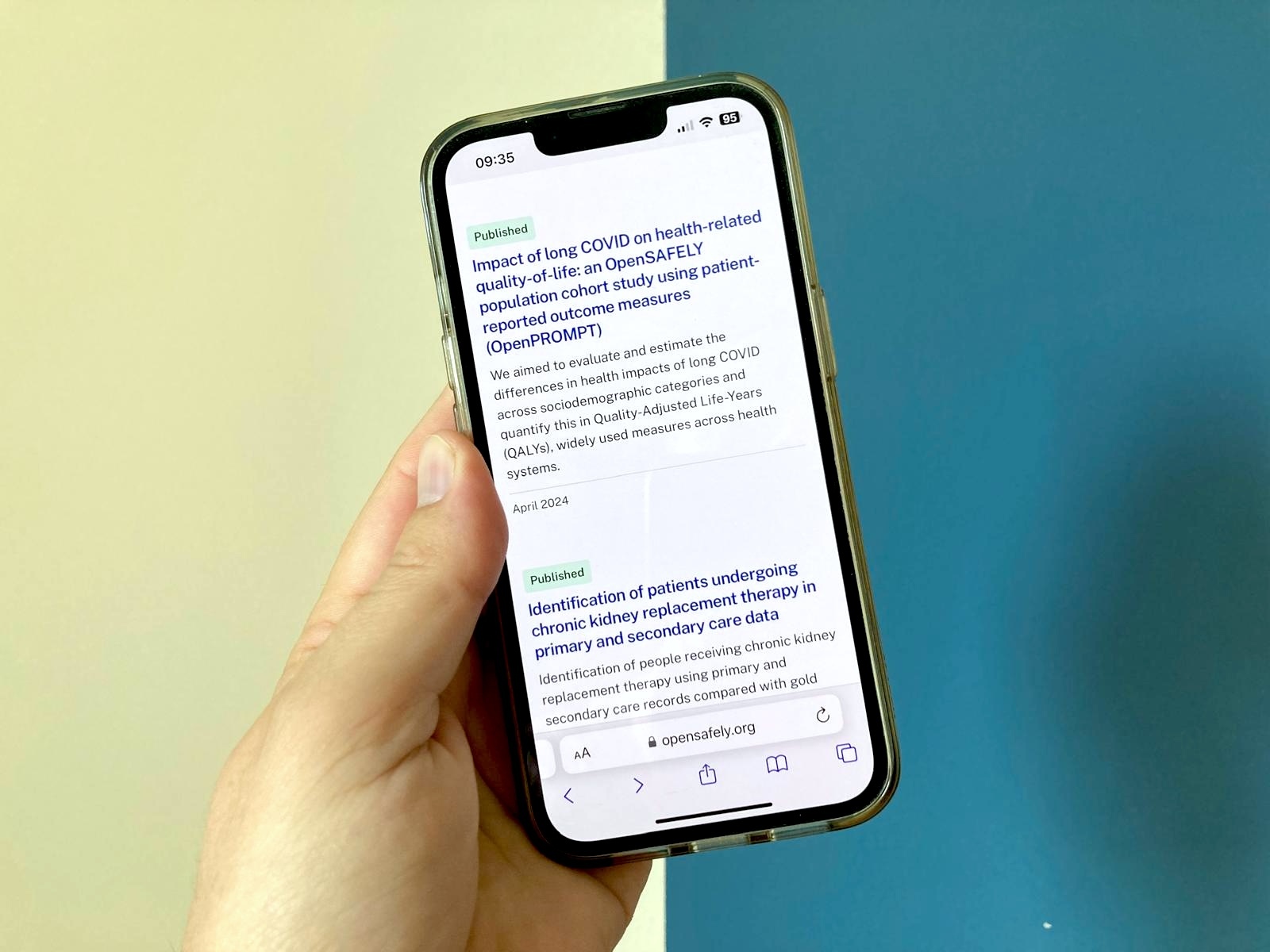
In the past 6 months we’ve been very productive at OpenSAFELY publishing 21 peer-reviewed papers on a variety of topics.

In this guest blog, Katie Bechman describes the proof-of-principle pharmacovigilance study she conducted with the help of her team from King’s College London, which used OpenSAFELY to examine the safety of sotrovimab, paxlovid and molnupiravir in prehospital treatment of Covid-19.

Two days of talks and workshops on research, data infrastructure, and open science.
The Research Experience team explain how they’ve been recording decisions

Most of the time, we like to keep things fairly formal on this blog - but if you’ll forgive us a small indulgence, today we’re going to blow our own trumpet a little. A couple of weeks ago, my colleague Rose Higgins and I went to an event in London where this Institute was awarded the Open Science Impact award, as part of the MRC Impact Prize. We were, needless to say, over the moon about it.
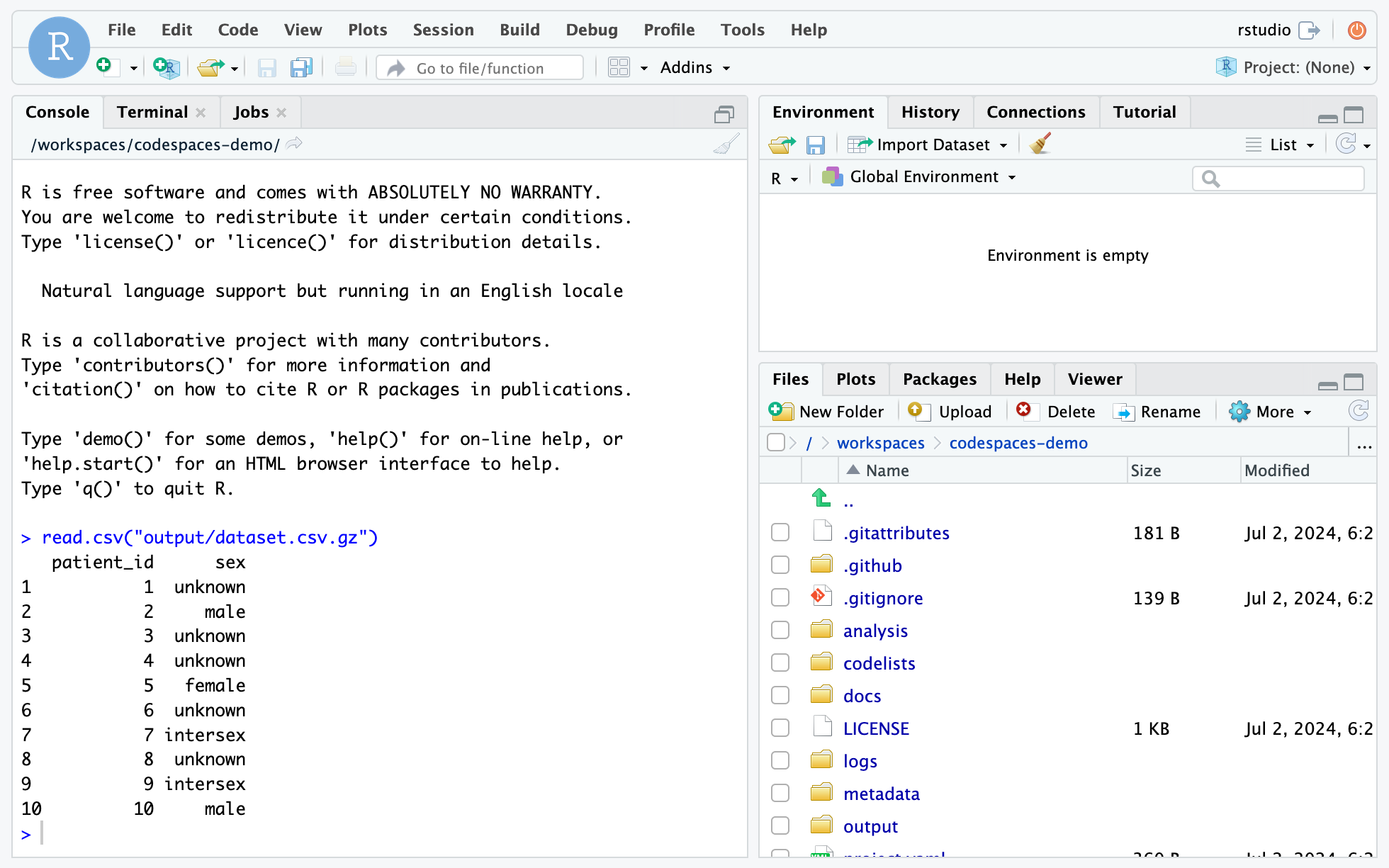
Spend more time answering your research questions – and less time wrangling your computer – with GitHub Codespaces and the OpenSAFELY development container.

Our new study looks at the rate that sick notes were issued for people with COVID-19, and how it changed over the course of the pandemic.
During the COVID-19 pandemic, there were no sustained increases in opioid prescribing.

We describe the features of OpenSAFELY that encourage and support reproducible research.

We describe some of our recent work investigating the impact of the COVID-19 pandemic on DMARD safety monitoring across >24 million patients’ records in England.

We’re hiring a research software advocate and we think you’d be a good fit

This newsletter contains all the latest updates from OpenPrescribing

We’re hiring software people and we’d love you to join us

In the second of a two-part blog series, pharmacists Chris Wood and Vicky Speed talk about the results of their research on medication review activity
In the first of a two-part blog series, pharmacists Chris Wood and Vicky Speed talk about designing their research on medication review activity

We’re hiring for our research teams

How we organize and plan work for our tech teams

Ben discusses the OpenSAFELY platform with fellow nerd Tim Harford

This newsletter contains all the latest updates from OpenPrescribing
Here we describe some of our recent work investigating the impact of the COVID-19 pandemic on safe prescribing using a set of quality assured indicators across 57 million patients’ records in England

In this guest blog, Mark Green describes their latest paper using OpenSAFELY.
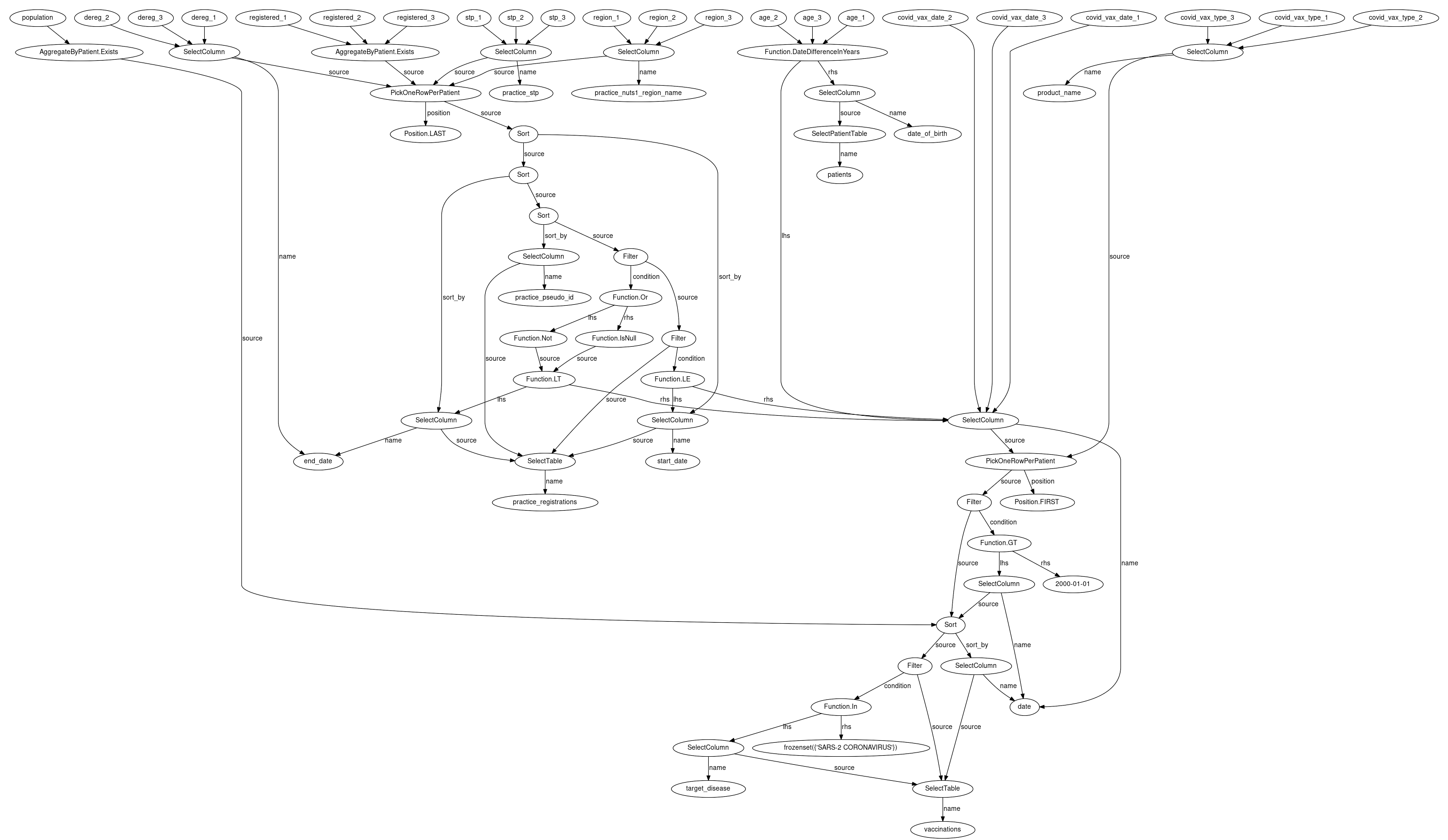
How can you be confident that the queries you write will be correctly interpreted and return the expected results?

To accompany the recent Ethnicity Short Data Report preprint we have created a short explainer for researchers using ethnicity in OpenSAFELY-TPP

The final session was a series of talks highlighting some of the research conducted in OpenSAFELY, some external perspectives on OpenSAFELY, and concluding remarks from our benefactor Peter Bennett, and director Ben Goldacre.

The third session in the Bennett Conference was a series of talks describing the overall operation of the OpenSAFELY platform and service.

A whistlestop tour of the whole history of our group, from OpenPrescribing via TrialsTracker to OpenSAFELY and on through Open Science, policy work, and more
The COVID-19 pandemic has reshaped many aspects of healthcare, including how antibiotics are prescribed in primary care settings. In this guest blog, Professor Diane Ashiru-Oredope lead pharmacist for antimicrobial resistance at UK Health Security Agency and our own Brian MacKenna, share some insights on repeat antibiotic prescribing and the link to health inequalities from a recent UK Health Security Agency analysis using OpenSAFELY.

We are delighted to share the announcement from NHS England and Department of Health and Social Care below, setting out the future of OpenSAFELY.
In this guest blog, Bang Zheng and Laurie Tomlinson from the London School of Hygiene and Tropical Medicine describe some of their lastest work on the comparative effectiveness and safety of currently recommended COVID-19 therapeutics in the community settings.

OpenPrescribing Autumn 2023 Newsletter.

In this guest blog, Mark Russell describes the research that he has undertaken with the help of his team from King’s College London using OpenSAFELY.

Xiaomin Zhong, Ya-Ting Yang, Dr Ali Fahmi, Dr Victoria Palin and Professor Tjeerd Van Staa from Manchester University have been using OpenSAFELY for analyses focused on the impact of COVID-19 on antimicrobial resistance since 2021. In this guest blog they describe some of their recent papers that have been published in peer review journals.

In this blog post Millie Green shares some of the findings from a federated analysis carried out using OpenSAFELY.
In this blog we explain what codelists are, how they are constructed and review existing literature on construction processes.
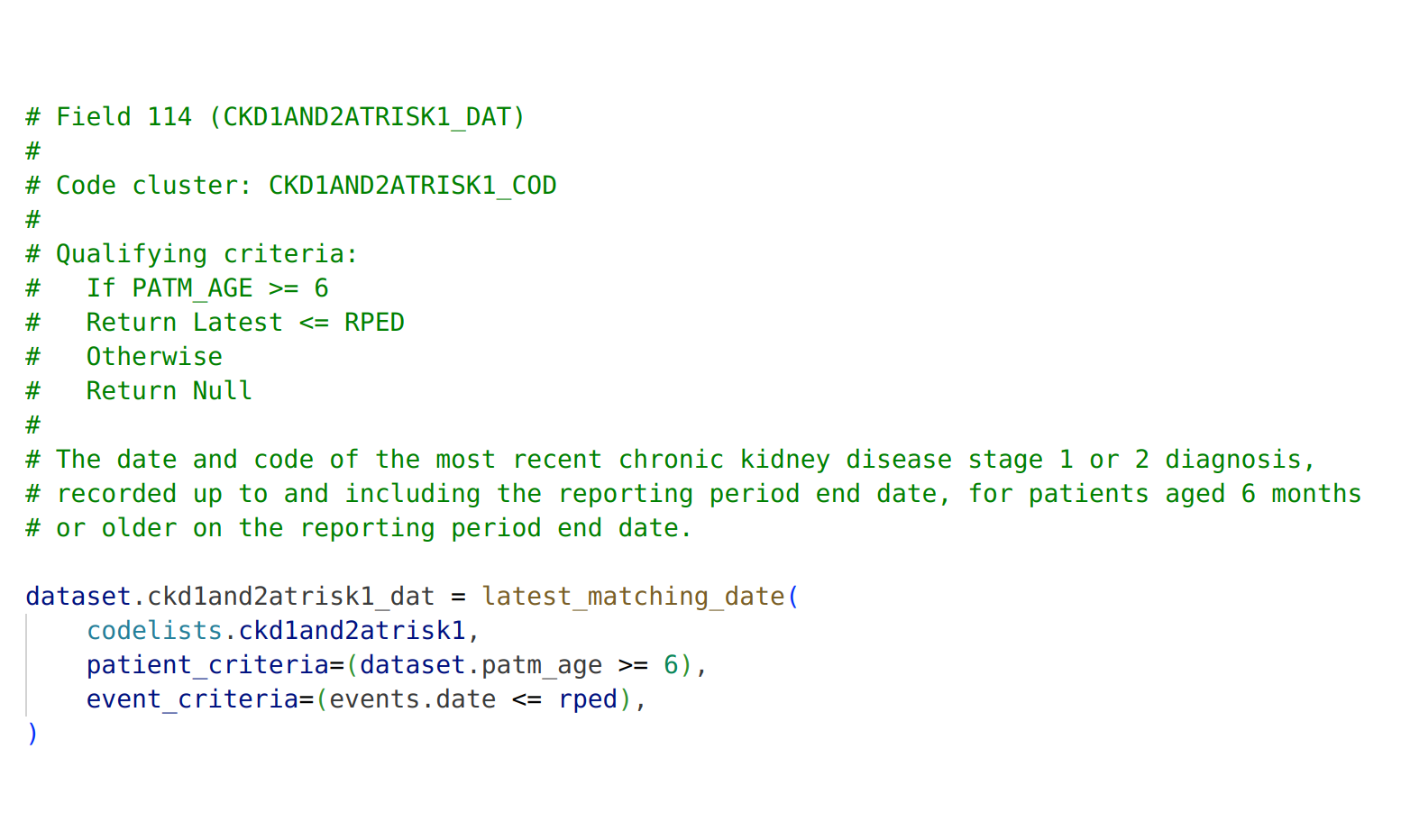
Why did we create a new query language?

The OpenSAFELY Collaborative has won a prestigious CogX Award for the Best Innovation in Open Source Technology

In this blog, we describe the development of a set of key measures used to monitor the ongoing impact of the COVID-19 pandemic on primary care as part of the OpenSAFELY Service Restoration Observatory (SRO). The results from this work have now been published in eLife.

Here we introduce the Bennett Institute’s Information Governance team, explaining who they are, and what they do.

Our new paper describes some of the biases that exist when estimating COVID-19 vaccine effectiveness using routinely-collected health data, and discusses the use of target trial emulation to avoid or mitigate these biases.

An introduction to what clinical codes are, why they are used and the terminology systems used within OpenSAFELY work.
A summary of our findings from running the OpenSAFELY output checking service.

Millie and Laurie share how some of the outputs from the work on COVID-19 therapeutics have been used to inform policy and guidelines in the NHS and beyond.
The latest paper from our NHS Service Restoration Observatory, examining changes in primary care activity over the pandemic was recently published in the British Journal of General Practice.

The OpenSAFELY Product Team’s mission is to understand our users’ needs, identify opportunities to improve OpenSAFELY products, and work with the tech teams to figure out the best solutions to build. Who are we? Catherine Stables is Lead Product Manager. In her previous role she was Data Product Manager for DataLoch, based at the University of Edinburgh. She has a background in academic research on cardiovascular disease, and a PhD from King’s College London.

Our OpenSAFELY study describing patterns in COVID-19-related mortality in the first five pandemic waves is now published open-access in the Lancet Public Health.

An outline the OpenSAFELY output checking service: who it involves, how our output checkers are trained and an overview of the output checking workflow.

Introducing the Bennett Institute’s Pipeline team, where we talk about who we are, what we do and some of the products we’re responsible for.

Here we introduce the Bennett Institute’s NHS Service Analytics team, explaining who they are, what they do, and why it is important.

Here we introduce the Bennett Institute’s Epidemiology team, explaining who they are, what they do, and why it is important.
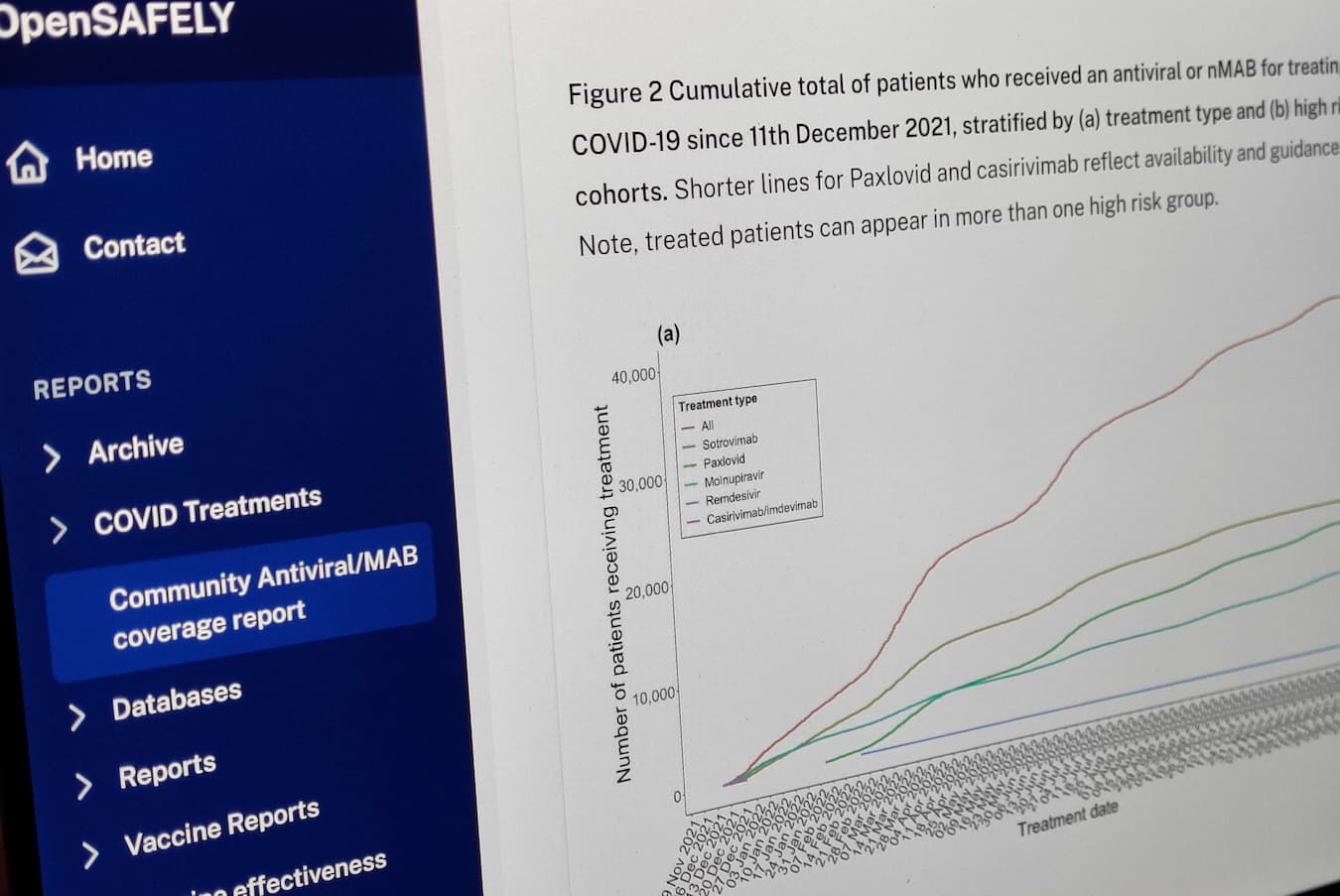
Our OpenSAFELY study comparing the Pfizer and Moderna vaccines for protecting against covid-19 during the first booster programme in England is now published open-access in the BMJ.

Here we introduce the Bennett Institute’s Clinical Informatics team, explaining what they do and how their experience in NHS clinical practice helps them do this.

Hello, from the Bennett Institute’s Data Team! In this short blog post, we’ll describe our mission, our backgrounds in industry and academia, and what we’re working on now.

Taking some time to describe the co-piloting programme in numbers, to give an idea of the scale of this programme, who our co-pilots are and who we are working with

Better and more efficient health science with reusable pipelines and cross-disciplinary expertise.

Taking a deeper look at the ‘Safe Outputs’ dimension of this framework, and how it is applied in OpenSAFELY.
Here we outline some of the research that has been produced with OpenSAFELY, as well as the features that make it such a great platform for research.
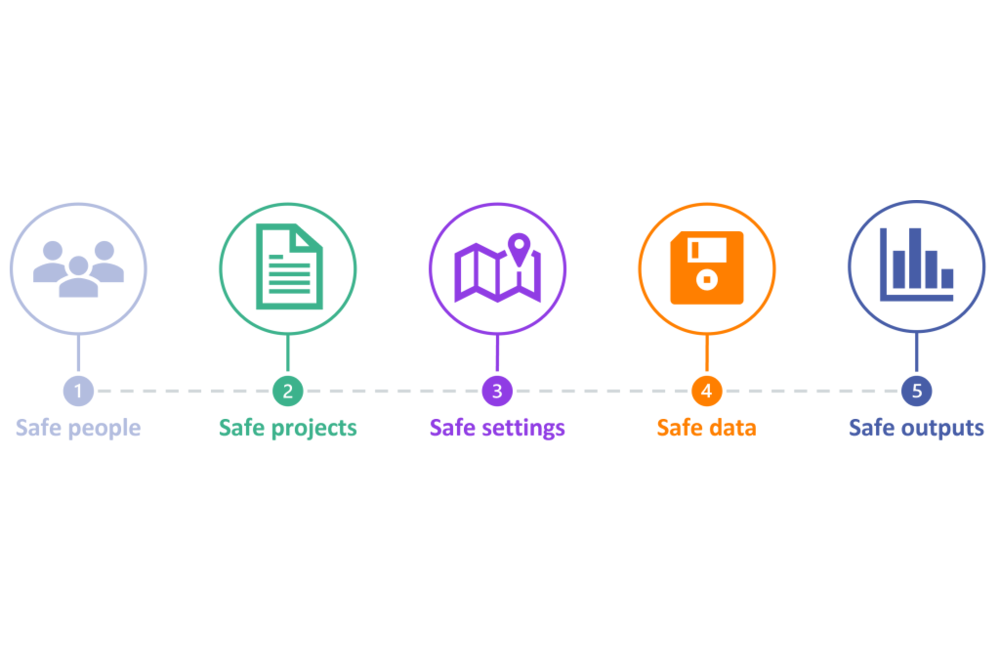
The Fives Safes framework is a popular framework for designing safe and efficient data access systems that has been adopted by a range of Trusted Research Environments (TREs) across the UK.
On 7th May 2020, the OpenSAFELY Collaborative pre-printed the world’s largest study into factors associated with death from COVID-19, based on an analysis running across the full pseudonymised health records of 40% of the English population.

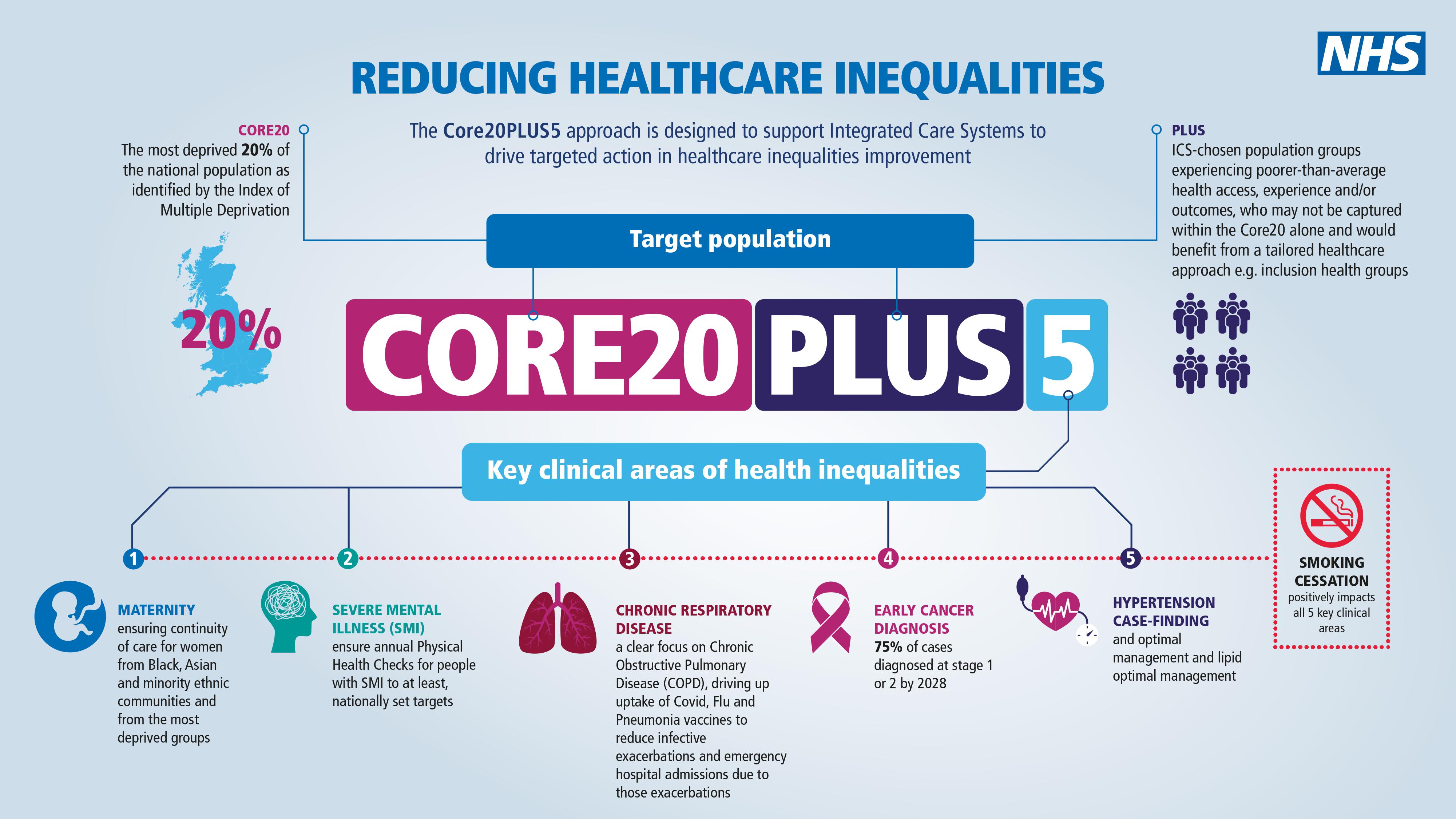
In order to tackle health inequalities NHS England have recently launched the Core20PLUS5 initiative to help local areas identify and reduce inequalities across key areas.

We recently presented on behalf of our team (grp-EHR) at an open science workshop, hosted by the MRC Biostatistics Unit at the University of Cambridge. This was a great opportunity for us to reflect on our team’s experiences using OpenSAFELY, and more broadly embracing open and team science approaches to our work.

As a team of co-pilots, we regularly take time to discuss how co-piloted projects are going and what we can do to help pilots and co-pilots make the most of their time together.

Rachel Seeley is the Head of Analytics at PrescQIPP CIC and has recently completed a project in OpenSAFELY on the safer use of anticoagulant medication. In this guest blog, Rachel describes her experience with using the OpenSAFELY platform and our co-pilot programme.

Bennett Institute resident pharmacist Brian MacKenna sets out some brief information on how we currently build medicines codelists on OpenSAFELY.

Our latest newsletter including information on: Bennett Institute for Applied Data Science, we are recruiting, Goldacre Review, updated Outlier Dashboards, recent changes to OpenPrescribing Measures, OpenSAFELY news, and new data news.

CIPHA and OpenSAFELY are hosting an introduction event for researchers in the North West of England who would like to work with CIPHA data using OpenSAFELY.

Our policy lead, Jess Morley, discusses the challenges involved in closing the gap in representation and reward for women working in these fields, and what we in the Bennett Institute are trying to do to help lower some of the associated barriers.

Our latest newsletter including information on: We are recruiting, recent changes to OpenPrescribing Measures, new Outlier Prescribing tool, maps update, recent research publications, OpenSAFELY news, and new data news.

I joined Bennett Institute in August 2021 to work as a data scientist on OpenSAFELY. This blog post describes my experience getting up and running with the OpenSAFELY pipeline.

This is a guest blog from the team at Cantabular, who have been exploring how their technology might fit into the OpenSAFELY ecosystem.

All new users of the OpenSAFELY platform get access to our supportive co-pilot programme, where each new OpenSAFELY user is assigned a member of the OpenSAFELY team as their co-pilot for the duration of their project.

This week sees the publication of an independent Citizens’ Jury commissioned for NHSx and the National Data Guardian which found that OpenSAFELY was by far the most strongly and consistently supported of all NHS COVID data projects examined.

On our first anniversary, from the Policy Lead in the Bennett Institute, this is the brief story of the positive side from all our lives: how OpenSAFELY came to life, and what we’ve achieved so far.

This is a draft discussion paper, the first of a series exploring “open team science” approaches to managing health data, and specifically how to create a collaborative computational data science ecosystem where the sharing and re-use of objects such as codelists and code is facilitated, encouraged, recognised, and rewarded. As a microcosm of this we have first explored “codelists”. There are currently no ‘answers’ or preferred solutions given. We will be holding an open discussion with the research community on 2nd March at 3pm - you can book to join us here.

We have been very busy since our last newsletter back in July and there are tonnes of exciting updates for you here! Measure Update: Total Oral Morphine Equivalence The Faculty of Pain Medicine has recently updated their recommendation on oral morphine equivalence (OME) which we use on our OpenPrescribing measure of OME. We have taken this opportunity to update and a new novel implementation of how we assess OME. Until this work is completed we have taken the decision to “suspend” the measure from dashboards however you can still view the old method using this link.

This is the code for the OpenSAFELY cohort extractor tool which supports the authoring of OpenSAFELY-compliant research, by: Allowing developers to generate random data based on their study expectations. They can then use this as input data when developing analytic models. Supporting downloading of codelist CSVs from the OpenSAFELY codelists repository, for incorporation into the study definition Providing tools to understand and visualise the properties of real data, without having direct access to it It is also the mechanism by which cohorts are extracted from live database backends within the OpenSAFELY framework.

This is the repository for the OpenSAFELY job runner. A job runner is a service that encapsulates: the task of checking out an OpenSAFELY study repo; executing actions defined in its project.yaml configuration file when requested via a jobs queue; and storing its results in a particular locations. The documentation is aimed at developers looking for an overview of how the system works. It also has some parts relevant for end users, particularly the project.

This is the code for the OpenSAFELY job server designed for mediating jobs that can be run in an OpenSAFELY secure environment. The Django app provides a simple REST API which provides a channel for communicating between low-security environments (which can request that jobs be run) and high-security environments (where jobs are run).

What is OpenSAFELY? Working on behalf of NHS England we have now built a full, open source, highly secure analytics platform running across the full pseudonymised primary care records of 24 million people, rising soon to 55 million, 95% of the population of England. We have pursued a new model: for privacy, security, low cost, and near-real-time data access, we have built the analytics platform inside the EHR data centre of the major EHR providers, where the data already resides; in addition we have built software that uses tiered increasingly non-disclosive tables to prevent researchers ever needing direct access to the disclosive underlying data to run analyses; code is developed against simulated data using open platforms before moving to the live data environment.

OpenPrescribing and Bennett Institute Papers It has been a busy month for paper publication at The Bennett Institute. We have written a brief description of the most recent papers below. Please sharewith colleagues and get in touch if you have any relevant observations! Remember you can read all our academic papers related to OpenPrescribing on our research page. Hospital medicines data: We are frequently contacted at OpenPrescribing about when we are going to make a hospital version.

Methotrexate Prescribing Safety — New paper in BJGP This week the British Journal of General Practice published our latest paper on unsafe prescribing of methotrexate. We found that the prevalence of unsafe methotrexate prescribing (10mg tablets) has reduced but remains common, with substantial variation between practices and CCGs. In the paper we also discuss recommendations for better strategies around implementation. Anyone can view the live data on unsafe methotrexate prescribing at openprescribing.

OpenSAFELY.org OpenSAFELY is a new secure analytics platform for electronic health records in the NHS, created to deliver urgent results during the global COVID-19 emergency. OpenSAFELY is a collaboration between the Bennett Institute, the EHR group at London School of Hygiene and Tropical Medicine and TPP who produce SystmOne. OpenSAFELY is now successfully delivering analyses across more than 24 million patients’ full pseudonymised primary care NHS records. The first analysis from OpenSAFELY is Factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients with more answers to important questions expected shortly.
OpenPrescribing.net has been updated this week with the latest release of prescribing data covering March 2020. In-depth analysis will be needed over the coming months, but this release gives us the first glimpse into the impact that COVID-19 has had on prescribing. At the Bennett Institute we have been quite busy with the new secure analytics platform OpenSAFELY but the following blog is a rapid analysis of the March prescribing data which others may find helpful to focus their own investigations.